Battery-Powered Balancing Robot DIY STEM Kit
$8.99$4.95
Posted on: Aug 8, 2023
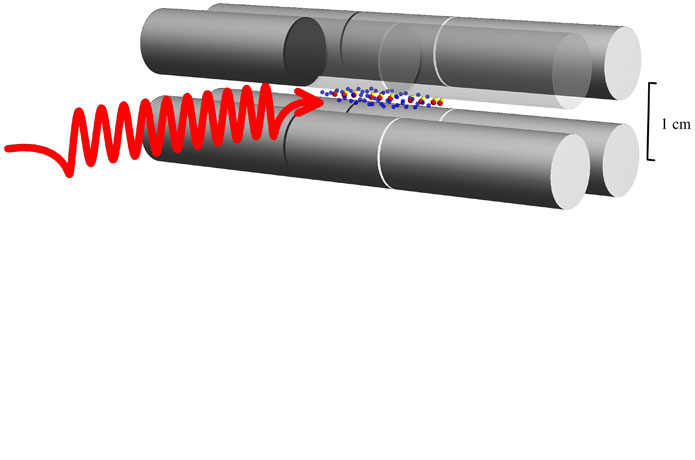
Using ultra-high-precision laser spectroscopy on a simple molecule, a group of physicists led by Professor Stephan Schiller Ph.D. from Heinrich Heine University Düsseldorf (HHU) has measured the wave-like vibration of atomic nuclei with an unprecedented level of precision. In the scientific journal Nature Physics, the physicists report that they can thus confirm the wave-like movement of nuclear material more precisely that ever before and that they have found no evidence of any deviation from the established force between atomic nuclei.
Simple atoms have been the subjects of precision experimental and theoretical investigations for nearly 100 years, with pioneering work carried out on the description and measurement of the hydrogen atom, the simplest atom with just one electron. Currently, hydrogen atom energies – and thus their electromagnetic spectrum – are the most precisely computed energies of a bound quantum system. As extremely precise measurements of the spectrum can also be made, the comparison of theoretical predictions and measurements enables testing of the theory on which the prediction is based.
Such tests are very important. Researchers around the world are seeking – albeit unsuccessfully to date – evidence of new physical effects that could occur as a result of the existence of Dark Matter. These effects would lead to a discrepancy between measurement and prediction.
By contrast with the hydrogen atom, the simplest molecule was not a subject for precision measurements for a long time. However, the research group headed by Professor Stephan Schiller Ph.D. from the Chair of Experimental Physics at HHU has dedicated itself to this topic. In Düsseldorf, the group has conducted pioneering work and developed experimental techniques that are among the most accurate in the world.
The simplest molecule is the molecular hydrogen ion (MHI): a hydrogen molecule, which is missing an electron and comprises three particles. One variant, H2+, comprises two protons and an electron, while HD+ comprises a proton, a deuteron – a heavier hydrogen isotope – and an electron. Protons and deuterons are charged “baryons”, i.e. particles which are subject to the so-called strong force.
Within the molecules, the components can behave in various ways: The electrons move around the atomic nuclei, while the atomic nuclei vibrate against or rotate around each other, with the particles acting like waves. These wave motions are described in detail by quantum theory.
The different modes of motion determine the spectra of the molecules, which are reflected in different spectral lines. The spectra arise in a similar way to atom spectra, but are significantly more complex.
The art of current physics research now involves measuring the wavelengths of the spectral lines extremely precisely and – with the help of quantum theory – also calculating these wavelengths extremely precisely. A match between the two results is interpreted as proof of the accuracy of the predictions, while a mismatch could be a hint for “new Physics”.
Over the years, the team of physicists at HHU has refined the laser spectroscopy of the MHI, developing techniques that have improved the experimental resolution of the spectra by multiple orders of magnitude. Their objective: the more precisely the spectra can be measured, the better the theoretical predictions can be tested. This enables the identification of any potential deviations from the theory and thus also starting points for how the theory might need to be modified.
Professor Schiller’s team has improved experimental precision to a level better than theory. To achieve this, the physicists in Düsseldorf confine a moderate number of around 100 MHI in an ion trap in an ultra-high vacuum container, using laser cooling techniques to cool the ions down to a temperature of 1 milli kelvin. This enables extremely precise measurement of the molecular spectra of rotational and vibrational transitions. Following earlier investigations of spectral lines with wavelengths of 230 µm and 5.1 µm, the authors now present measurements for a spectral line with the significantly shorter wavelength of 1.1 µm in Nature Physics.
Professor Schiller: “The experimentally determined transition frequency and the theoretical prediction agree. In combination with previous results, we have established the most precise test of the quantum motion of charged baryons: Any deviation from the established quantum laws must be smaller than 1 part in 100 billion, if it exists at all.”
The result can also be interpreted in an alternative way: Hypothetically, a further fundamental force could exist between the proton and deuteron in addition to the well-known Coulomb force (the force between electrically charged particles). Lead author Dr Soroosh Alighanbari: “Such a hypothetical force may exist in connection with the phenomenon of Dark Matter. We have not found any evidence for such a force in the course of our measurements, but we will continue our search.”
 'The atomic bomb ... made the prospect of future war unendurable. It has led us up those last few steps to the mountain pass; and beyond there is different country.'
'The atomic bomb ... made the prospect of future war unendurable. It has led us up those last few steps to the mountain pass; and beyond there is different country.'